OTC: Over the Counter Hearing Aids – Breaking News
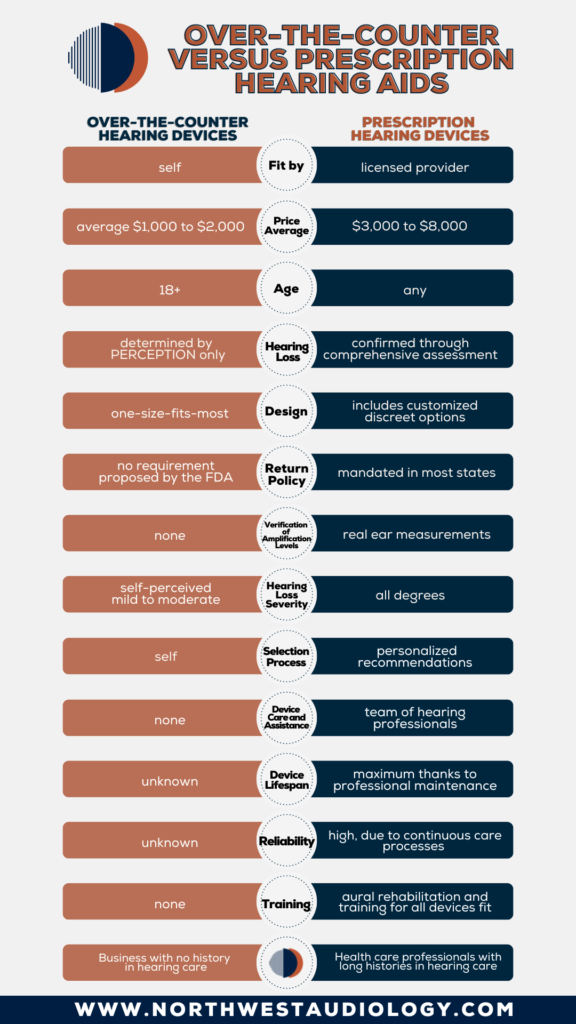
In 2017 Congress passed legislation to a create a category of over-the-counter hearing aids (OTCs). While OTCs have existed for quite some time, this legislation provides framework for what constitutes an OTC device. Please see below about who, and who might not, benefit from OTC devices. And remember, the first step is a comprehensive hearing evaluation from a licensed Audiologist to rule out any medical concerns.
Who is a candidate for OTCs?
- Those with perceived mild to moderate hearing loss
- Those who feel comfortable setting the device to their perceived hearing level via a smartphone
- Those who are over the age of 18
Who might not be a candidate for OTCs?
- Those with tinnitus (ringing, buzzing, rushing, roaring, cricket sounds in ears)
- Those who hear better in one ear than the other
- Those with a sudden onset of hearing loss or rapidly changing hearing loss
- Those taking ototoxic medications or a history of chemotherapy treatment
- Those with drainage or pain in their ears
- Those with dizziness or vertigo
Regardless of which category you fit into, the FDA is still recommending a comprehensive hearing evaluation. If you decide to proceed with OTC devices, we are offering verification and cleaning services. If you have purchased OTC devices and need assistance with device orientation, we can assist with that as well. Please call us to schedule whatever you may need, from a comprehensive evaluation and device selection, to verification and evaluation of your OTC devices.